Seed Grant Program
This program offers internal funds to support and grow collaborative One Health research projects across the University of Tennessee system. The goal of the program is to create transdisciplinary synergies among faculty, staff, students, and external collaborators that embrace a One Health approach to investigations.
2023 Seed Grant Recipients
With financial support from the UT College of Veterinary Medicine Center of Excellence, Tennessee RiverLine*, and the UT Humanities Center**, the One Health Initiative offered three $40,000 awards to multidisciplinary teams tackling complex issues requiring a One Health approach.
- PI: Kimberly Gwinn (Herbert College of Agriculture, Department of Entomology and Plant Pathology)
- Co-PI: Julia Albright (College of Veterinary Medicine, Small Animal Clinical Sciences)
- PI: Sarah Bolivar (College of Architecture and Design, School of Landscape Architecture)
- Co-PI: Michael Ross (Herbert College of Agriculture, Department of Plant Sciences; College of Architecture and Design, School of Landscape Architecture)
- Co-PI: Jason Brown (College of Arts and Sciences, School of Art)
- PI: Stephen Collins-Elliott (College of Arts and Sciences, Department of Classics)
- Co-PI: Alison Damick (McClung Museum of Natural History and Culture)
2022 Seed Grant Recipients
In 2022, the One Health Initiative offered several small awards (up to $40,000), including one exploring healthy, living shorelines on the Tennessee River (*in partnership with Tennessee RiverLine) and one globally-focused award addressing one or more of the United Nations Sustainable Development Goals (**in partnership with UT’s Smith Center for International Sustainable Agriculture and the Center for Global Engagement).
- PI: Madhu Dhar (College of Veterinary Medicine, Large Animal Clinical Sciences)
- Co-PI: Dayakar Penumadu (Tickle College of Engineering, Department of Civil and Environmental Engineering)
- PI: Shigetoshi Eda (Herbert College of Agriculture, Department of Forestry, Wildlife, and Fisheries)
- Co-PI: Doris D’Souza (Herbert College of Agriculture, Department of Food Science)
- Co-PI: Jayne Wu (Tickle College of Engineering, Department of Electrical Engineering and Computer Science)
- PI: Michael McKinney (College of Arts and Sciences, Department of Earth and Planetary Sciences)
- Co-PI: Andrea Ludwig (Herbert College of Agriculture, Department of Biosystems Engineering and Soil Sciences)
- Co-PI: John Schwartz (Tickle College of Engineering, Department of Civil and Environmental Engineering)
- Co-PI: Michael Ross (Herbert College of Agriculture, Department of Plant Sciences)
- Co-PI: Garrett Ferry (Facilities Services)
- PI: Jennifer Retherford (Tickle College of Engineering, Department of Civil and Environmental Engineering)
- Co-PI: Nan Gaylord (College of Nursing)
- Co-PI: Sara Mulville (Smith Center for International Sustainable Agriculture)
- Co-PI: David Ader (Smith Center for International Sustainable Agriculture)
Abstract: One Health challenges are crosscutting and complex. An important step toward advancing a One Health global strategy (eg. Global Health Security Agenda (13)) requires building relevant One Health core competencies (3,6,7) in the next generation. We propose to develop a One Health international training program to equip students with considerable knowledge and skills to complement their own areas of expertise. We will adopt numerous evidence-based educational training modules and frameworks to build students’ core competencies (e.g. USAID One Health Core Competency; CDC Safe Water, Liberating Structures, etc.) (2,5,8,10,14). Strong emphasis will be placed on promoting systems-thinking approaches and multi-stakeholder engagement throughout the pilot project life cycle. Project activities will help students analyze, apply, and adapt tools from different disciplines to solve water-related challenges using a One Health approach. Root causes of waterborne zoonoses will be evaluated and community-based interventions applied within the context of the Emberá-Wounaan comarcas. We propose to utilize a One Health approach, based on the Carnegie Foundation academic outreach and engagement principles (18), and work with local stakeholders to address UN Sustainable Development Goals (SDG), namely Goal 3 (Good Health and Wellbeing) and Goal 6 (Clean Water and Sanitation) in the indigenous community of Las Lajas, Darien Province. This intervention will serve as a case study for One Health approaches and provide students with experiential learning opportunities to apply their specific disciplines within this framework.
- PI: Debasish Saha (Herbert College of Agriculture, Department of Biosystems Engineering and Soil Sciences)
- Co-PI: Subhadeep Chakraborty (Tickle College of Engineering, Department of Mechanical, Aerospace, and Biomedical Engineering)
- PI: Timothy Truster (Tickle College of Engineering, Department of Civil and Environmental Engineering)
- Co-PI: Pierre-Yves Mulon (College of Veterinary Medicine, Large Animal Clinical Sciences)
- Co-PI: David Anderson (College of Veterinary Medicine, Large Animal Clinical Sciences)
Abstract: Many challenges are faced by clinicians and researchers when making decisions regarding bone injury and the ability to restore normal form and function to the bone. Translational research focused on orthopedic applications such as biomedical implants carries substantial value, as numbers of individuals requiring orthopedic implants are continuously increasing. This study will develop and combine physics-based and machine-learning models for assessing the effect of bone defects using the goat tibia as a model. The finite element phase field fracture model provides a high-fidelity virtual laboratory to map 3D specimen scans into bone strength and microcrack profiles, and the Gaussian Process Regression (GPR) model provides a low-fidelity fast-sampling platform for optimizing the defect placement within a new specimen to maximize strength and minimize deleterious effect on bone health. Combining high- and low-fidelity models enables a nearly unlimited number of variations to be analyzed prior to undertaking a research study, aiding in the design of novel implants or in refinements of orthopedic surgery. Outcomes of this study would improve in vivo studies in animals, achieving the goal of reduce, refine and replace in animal research. Computational models also will enhance investigations of surgical techniques to improve the healing of defects in injured patients. Our long-term goals are to develop methods for computational estimation of the effect of risk factors, modifications to surgical model designs, and development of new surgical implants and approaches while reducing the reliance on first ex vivo and second in vivo pilot study modeling, thereby conserving resources.
Publications generated by this project:
- Debangshu, P., P. Mulon, Z. Arwood, D. Penumadu, and T. Truster. In prep. Method for CT voxel-based finite element analysis and validation with digital image correlation system. MethodsX.
2021 Seed Grant Recipients
- PI: Michelle Dennis (College of Veterinary Medicine, Department of Biomedical and Diagnostic Sciences)
- Co-PI: Nina Fefferman (College of Arts and Sciences, Department of Ecology and Evolutionary Biology and Department of Mathematics)
- Co-PI: Gerald Dinkins (McClung Museum of Natural History and Culture)
- Co-PI: Rebecca Hardman (Herbert College of Agriculture, Department of Forestry, Wildlife, and Fisheries)
- Co-PI: Gus Engman (Herbert College of Agriculture, Department of Forestry, Wildlife, and Fisheries)
Freshwater mussels are key species for riverine ecosystem health because of their indispensable roles in water purification and nutrient cycling. However, over the last several decades, mussel populations have been severely impacted across North America by poorly-understood mortality epidemics. Since 2016, seasonal mortality events have decimated mussels of the upper Clinch River, a location treasured for unique mussel biodiversity, including 20 federally endangered species. This study aims to determine likely causes of mortality in the Clinch River with an in-situ experiment that measures seasonal changes in the health of sentinel mussels maintained in silos at impacted sites. Observations of growth and survivorship, clinical signs of disease, hemolymph indices, histopathology, and bacterial microbiome shifts during the experiment will be paired with population dynamic data from previous mortality events. These data will help build predictive models considering observed spatiotemporal patterns and likely causative agents to draw conclusions about causes of mussel mass-moralities.
- PI: Matthew Gray (Herbert College of Agriculture, Department of Forestry, Wildlife, and Fisheries)
- Co-PI: Neelam Poudyal, (Herbert College of Agriculture, Department of Forestry, Wildlife, and Fisheries)
- Co-PI: Nina Fefferman (College of Arts and Sciences, Department of Ecology and Evolutionary Biology and Department of Mathematics)
The regional, national, and international trade of wildlife has facilitated the movement, spillover, and global emergence of numerous infectious diseases including SARS-CoV-2, viral hemorrhagic septicemia virus, and chytrid fungi. Wildlife pathogens and zoonoses have cost global economies trillions of dollars and substantial human life and biodiversity loss, leading to increasing calls to regulate or prohibit the trade of exotic wildlife. The wildlife trade industry exists as a network of nodes which can amplify or dampen pathogen dynamics and thus affect spillover risk. Each node in the network has their own economic and psychosocial values that affect their behaviors and practices, which in turn affect network behavior and subsequent pathogen dynamics. Further, consumers and government can affect network behavior via product demand, perceived values of biodiversity, and regulations or policies. This bidirectionally coupled system of spatially explicit disease processes and socioeconomic feedbacks in a hierarchical network presents unique challenges and novel opportunities to understand pathogen transmission and spillover risk. We hypothesize that socioeconomic incentives targeting industry and consumer behavior will alter emergent structural properties of a live animal trade network and influence patterns in pathogen dynamics. We will identify incentive structures that motivate nodes to alter behaviors and decrease pathogen spread in industry and spillover risk to wild populations. Understanding the social and economic incentives that shape the topology of disease networks requires a multi-scale approach that considers node and network factors driven by socioeconomic behavior, such as changes in consumer demand for particular species. Our overarching task is to characterize the amphibian trade network in the US by considering disease processes across two hierarchical scales with possible socioeconomic feedbacks.
Publications generated by this project:
- Cavasos, K., N. C. Poudyal, J. L. Brunner, A. R. Warwick, J. Jones, N. Moherman, M. George, J. D. Willard, Z. Brinks, and M. J. Gray. 2023. Attitudes and behavioral intentions of pet amphibian owners about biosecurity practices. EcoHealth, https://doi.org/10.1007/s10393-023-01645-8.
- Cavasos, K., N. C. Poudyal, J. L. Brunner, A. R. Warwick, J. Jones, N. Moherman, M. George, J. D. Willard, Z. T. Brinks, and M. J. Gray. 2023. Exploring business stakeholder engagement in sustainable business practices: Evidence from the US pet amphibian industry. Business Strategy and the Environment, DOI: 10.1002/bse.3455.
- Cavasos, K., R. K. Adhikari, N. C. Poudyal, A. R. Warwick, and M. J. Gray. 2023. Natural area visitors’ potential role in preventing pathogen threats to amphibian biodiversity. Environmental Conservation 50: 142 – 147. DOI: https://doi.org/10.1017/S0376892923000048.
- Cavasos, K., R. K. Adhikari, N. C. Poudyal, A. R. Warwick, and M. J. Gray. 2023. Natural area visitors’ willingness to pay for amphibian conservation in a global biodiversity hotspot. Journal for Nature Conservation 76, 126499, https://doi.org/10.1016/j.jnc.2023.126499.
- Cavasos, K., R. Adhikari, N. C. Poudyal, J. L. Brunner, A. Warwick, and M. J. Gray. 2023. Understanding the demand for and value of pathogen-free amphibians to US pet owners. Conservation Science and Practice, e12995.
- Wright, J., R. Almeida, M. J. Gray, M. Bletz, J. Lockwood, S. Masecar, J. Piovia-Scott, A. Warwick, and N. Fefferman. In review. Trade network surveillance strategies. Ecosphere.
- PI: Jun Lin, (Herbert College of Agriculture, Department of Animal Science)
- Co-PI: Qiang He (Tickle College of Engineering, Department of Civil and Environmental Engineering)
Escherichia albertii, often misidentified as Escherichia coli, has become an emerging human enteric pathogen. However, important animal and environmental reservoirs of this pathogen are still not clear. Based on recent preliminary studies, we hypothesize that chickens and raccoons are unique and significant players in the dynamic interactions among the enteric E. albertii pathogen, animals, humans, and their shared environment. To test this, we will perform a large scale study by pursuing following two aims: 1) examine the prevalence of E. albertii in US poultry production, wild raccoons, and surface waters; and 2) characterize the isolated E. albertii together with US human E. albertii strains using a panel of microbiological and genomics approaches. Completion of this project will provide insights into the ecology, evolution and transmission of E. albertii, and help us control the emerging E. albertii in a complex ecosystem using One Health approach.
Publications generated by this project:
- Hinenoya, A., X.P. Li, X. Zeng, O. Sahin, R. A. Moxley, C. M. Logue, B. Gillespie, S. Yamasaki, J. Lin. 2021. Isolation and characterization of Escherichia albertii in poultry at the pre-harvest level. Zoonoses and Public Health. 68:213-225.
- Hinenoya, A., H. Wang, E.M. Patrick, X. Zeng, L. Cao, X.P. Li, R.L. Lindsey, B. Gillespie, Q. He, S. Yamasaki, J. Lin. 2022. Longitudinal surveillance and comparative characterization of Escherichia albertii in wild raccoons in the United States. Microbiological Research. 262:127109.
- Wang, H., L. Zhang, L. Cao, X. Zeng, B. Gillespie, J. Lin. 2022. Isolation and characterization of Escherichia albertii originated from the broiler farms in Mississippi and Alabama. Veterinary Microbiology. 267:109379.
- PI: Chunlei Su (College of Arts and Sciences, Department of Microbiology)
- Co-PI: Richard Gerhold (College of Veterinary Medicine, Department of Biomedical and Diagnostic Services)
- Co-PI: Michelle Dennis (College of Veterinary Medicine, Department of Biomedical and Diagnostic Services)
- Co-PI: Sree Rajeev (College of Veterinary Medicine, Department of Biomedical and Diagnostic Services)
Transmission of zoonotic diseases is affected by multiple transboundary factors including humans, animals, plants and the environment. Understanding the interplay of these factors is pivotal for the prevention and mitigation of these diseases. One essential step to reach this goal is to detect and identify zoonotic pathogens in clinical animals, reservoir hosts, and the environment, which provides critical information for surveillance. Currently, there is lack of an integrated system to investigate and conduct surveillance of multiple zoonotic pathogens simultaneously. Our One Health Initiative Research project is to address this problem by developing an integrated system to detect and identify zoonotic pathogens. This system aims to detect novel and known zoonotic pathogens from animals and the environment by metagenome sequencing and multiplex genotyping approaches. We expect this system will be highly scalable for surveillance of a large number of pathogens from animals, plants, humans and environment in the near future.
- PI: Brian Whitlock (College of Veterinary Medicine, Large Animal Clinical Sciences)
- Co-PI: Bhavya Sharma (College of Arts and Sciences, Department of Chemistry)
- Co-PI: Allison Renwick (College of Veterinary Medicine, Comparative and Experimental Medicine Program)
Animals and humans frequently experience inflammation. Obesity has become a pandemic, causing chronic inflammation and impaired reproduction in humans. Lipopolysaccharide (LPS; endotoxin) is produced by bacteria and is responsible for the initiation of inflammation in obesity. The mechanism for impairment of reproduction in obese humans and animals likely involves reduction in neuropeptides in the hypothalamus . However, most research uses an acute model of inflammation; thus, there is a great need to develop a model of chronic inflammation. A valuable comparative model to evaluate the response to LPS in humans is sheep. They are similar in size, easy to handle, have large amounts of blood and tissue for collection and are physiologically similar. The focus of this study is to develop an animal model, using sheep, of chronic inflammation to determine its effects on hypothalamic neurons [KNDy (kisspeptin-neurokinin B-dynorphin) neurons] controlling reproduction.
- PI: Xinhua Yin (Herbert College of Agriculture, Department of Plant Sciences
- Co-PI: Joshua Fu (Tickle College of Engineering, Department of Civil and Environmental Engineering)
Influences of climate change on crop production are largely unknown in Tennessee, the USA, and the world. Cotton is a major crop in Tennessee and beyond. Forecasting of cotton yield under climate change is considered an advanced tool for future planning to assure global fiber security. The objective of this project is to assess the impacts of climate (temperature, precipitation) changes under two climate scenarios (baseline RCP4.5 and RCP8.5) on cotton production and estimate the performances of sustainable management practices under climate change via computational simulation. An existing long-term cotton field experiment in Jackson, TN will be used. This experiment was initiated in 1981 and has so far been continually conducted for 40 years. The state-of-the-art DSSAT computational model will be used. Through Extensional education, farmers will recognize the impacts of climate change on future cotton production, and will adopt relevant best management practices in cotton production under climate change.
One Health Research at UT
Below is a sampling of One Health research projects at UT; it is not a comprehensive list.
(UT Faculty and Researchers: To have your project(s) added to the list, contact us at onehealth@utk.edu!)
Human Health
Researchers: Xueping Li, Department of Industrial and Systems Engineering,
Elizabeth B. Strand, Colleges of Veterinary Medicine and Social Work
In this project, we will utilize a dispersed professional peer response model to connect at risk and suicidally ambivalent DVM’s through the development of a technology platform: the Life Boat web app. This technology allows to encourage, empathize, empower and support users to seek help and reduce stigma in being suicidal. We will form a Life Boat team by connecting at risk DVM with trained DVM mentors with “lived experience” and mental health coach. A function rich front-end and an intelligent back-end will be developed through the convergent research of social work, mental health, systems engineering, and design thinking.

Researcher: Charles Sims, Howard H Baker Jr Center for Public Policy
Using currently available data we develop a benchmark scenario that finds that a publicly-imposed social distancing rule to curb the spread of COVID-19 ought to be implemented for 12 weeks. We also identify alternative scenarios where the shorter duration social distancing programs seen throughout the United States may be efficient. Our approach is novel in that it accounts for uncertainty in transmission of the disease and the potential for permanent economic effects of social distancing rules. The social distancing rule is treated as an asset whose benefit is uncertain due to the inability to predict the evolution of the disease. The novel features of our approach allow us to draw two conclusions about the efficient timing of public social distancing programs in response to COVID-19.
First, uncertainty in transmission leads to a risk premium that creates a modest incentive to delay closing and reopening the economy. Second, hysteresis in economic impacts of social distancing leads to hysteresis in the efficient time to reopen the economy. Because reopening results in a second wave of infections that may be worse than expected and social distancing rules create permanent economic impacts, it is efficient to delay reopening the economy longer than suggested by benefit-cost analyses of social distancing programs. In our benchmark scenario, this bias results in reopening 27 days too soon.
Researcher: Heather Sedges, Department of Family and Consumer Sciences
During times of high and chronic stress it may seem impossible to figure out where to even start, what to ask for help with, or where to turn. The idea of locating supportive community-based resources and navigating access to them is a daunting task even for those without increased pandemic-related stress. Participants assigned a UT FRIEND will engage in an initial needs assessment. The UT FRIEND will then match needs to available community-based resources, create a plan to engage those resources, and reconnect to reassess needs as they develop, and hopefully diminish.
Graduates of the UT FRIENDS program will have the opportunity to “pay it forward” through an information/resource exchange where they can volunteer their own abilities and time to support the needs of others in their community. Through the UT One Health Initiative (OHI) this program ensures collaborative pathways to resiliency.
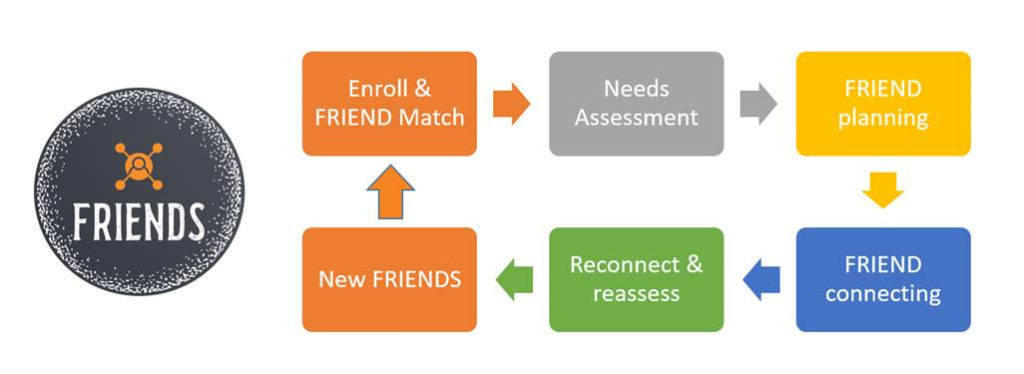
Researcher: Heather Sedges, Department of Family and Consumer Sciences
Agriculture in the United States is in a downturn, with significant losses over the past five years resulting in farm and ranch closures, forfeitures, and dramatically rising rates of farm and ranch stress. Farms and ranches in the southern region of the United States (as listed in the RFA: Alabama, Arkansas, Florida, Georgia, Kentucky, Louisiana, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, Virginia, Puerto Rico, and the Virgin Islands) are particularly vulnerable to the aforementioned issues contributing to increased farm and ranch stress, and are therefore the region of focus for this proposal. This project’s primary audiences are farmers and ranchers, as well as those working proximal to them such as Cooperative Extension Agents, lenders, commodity organizations, agricultural retailers, as well as medical and mental health professionals.
To this end, we propose working in a collaborative manner that ensures that all parties have a voice in decision-making, while also moving toward collective goals of establishing a hotline, website, resource clearinghouse, e-academy, and support groups. These objectives will be accomplished through dedicated action teams and national partners standing ready to connect farmers to existing and created resources and programs. With an established network of over 50 partners, we are ready and able to respond in meaningful and sustainable ways to the needs of farmers and ranchers in the southern region.
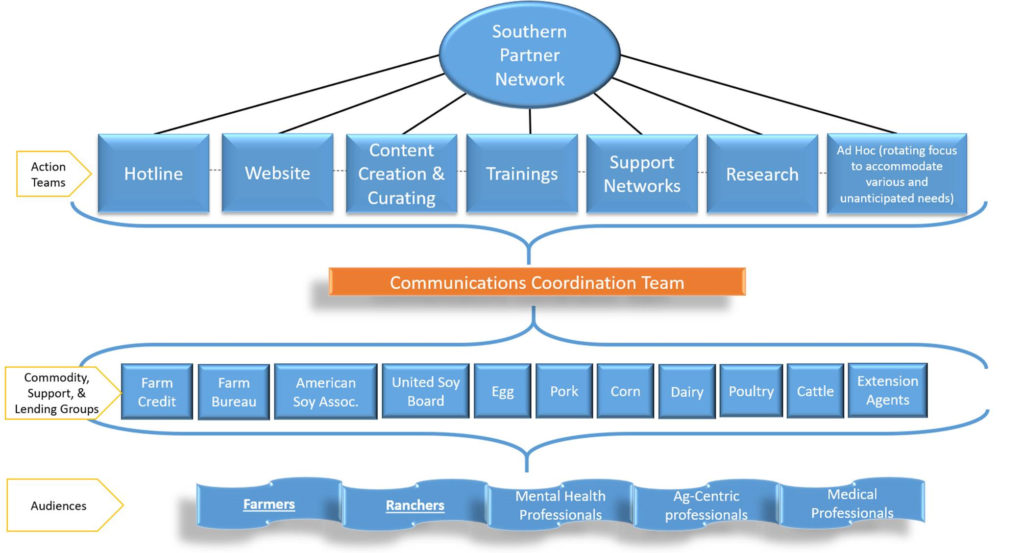
Researcher: Laura E. Miller, College of Communication and Information
This project explores the ways in which people seek information and manage uncertainty about COVID-19. Through in-depth interviews, we are interested in the sources of uncertainty that people are experiencing and how such uncertainties are managed.
Researcher: Laura E. Miller, College of Communication and Information
Young adult cancer survivors may experience unique interactional challenges as they navigate cancer treatment and beyond. Through an exploration of survivors’ and network members’ perspectives, this project aims to better understand the patterns of disclosure and information sharing among young adults and their loved ones.
Researcher: Laura E. Miller, College of Communication and Information
How do interprofessional health care teams manage uncertainty in the ever-changing emergency department setting? This project utilizes observational methodologies to gain a better understanding of how interprofessional health care teams communicate amid uncertain conditions.
Researcher: Laura E. Miller, College of Communication and Information
This project explores the ways in which couples communicate and support each other as they navigate opioid use disorder. Communication patterns between the couple and their families are explored through in-depth interviews with both members of the couple.
Researchers: Xueping Li, Department of Industrial and Systems Engineering
Lisa Merritt, College of Nursing
Motivation: Telehealth, virtual care platforms to connect healthcare providers and patients by phone or video chat, has emerged to transform healthcare delivery in the past decade by providing an alternative to in-person office visit. The current COVID-19 crisis suddenly makes it the new norm for non-emergent patients and “an indispensable tool” to help “flatten the curve of infections” (Dr. Lee Schwamm, Harvard Health Blog, March 24, 2020). Not only telehealth helps free up medical staff and equipment needed for those seriously ill from COVID-19, it also keeps people from congregating in small spaces like waiting rooms and keeps healthcare providers from patients and providers as “medical distancing”. However, most healthcare providers have very limited training on how to effectively engage patients and adapt to this new way of providing healthcare services without a hands-on physical exam.
Solution: We developed a Virtual Visit Simulation (V-Visit Sim) App that replicates a virtual visit encounter between a healthcare provider and a patient. The purpose is to train healthcare providers through simulated telehealth cases (encounters) in which healthcare providers and patient/caregivers interact with each other through a chat-message format. It can be expanded to video chat via standardized patients (i.e., sample patients or simulated patients). A chat-bot engine in the backend will respond to relevant questions or “I am not sure”/”I don’t know” to irrelevant questions. The goal is for providers to collect appropriate information from the history and virtual physical exam (chat messages, images, and video) and develop a correct diagnosis and management plan.

Researcher: Rebecca Trout Fryxell, Department of Entomology and Plant Pathology
La Crosse encephalitis (LACE) is the most common pediatric arthropod-borne disease in the United States and is an emerging infectious disease in the southern Appalachian region. LACE is caused by infection with the La Crosse virus (LACV), which is transmitted by the bite of infected Aedes mosquitoes. Symptoms usually manifest in children, and complications associated with the disease can be fatal. Because there is no preventive vaccine for LACE, the only effective method for reducing morbidity is the reduction of contact between humans and host-seeking mosquitoes, and because of a lack of sufficient vector control infrastructure, raising community awareness remains the primary method of prevention. As a way to do this, we are collaborating with many residents of east Tennessee (including middle and high school educators) to develop a community-driven mosquito surveillance program. Data obtained through these efforts are used to study the distributions of native and invasive Aedes mosquitoes in order to identify areas with increased risk for LACV transmission where novel small-scale vector control measures may be implemented.
Zoonoses
Researchers: Shigetoshi Eda, Department of Forestry, Wildlife & Fisheries and
Jayne Wu, Department of Electric Engineering and Computer Science
It is estimated that over 70% of emerging infectious diseases are caused by pathogens that infect both humans and animals (i.e. zoonotic diseases). As such pathogens survive in the environment (surface, water, soil, and air) staying infectious, it is imperative to control the pathogens in humans, animals, and the environment. Multilateral collaboration with One Health approach is therefore required for the prevention and control of zoonotic diseases.
An immediate example of such zoonotic diseases is COVID-19. The causative agent of COVID-19, SARS-CoV-2, is a zoonotic pathogen that transmits from wildlife animals to humans. The recent finding showed that the virus could infect domestic animals as well and that stayed active in the environment for hours or even days. Thus, detection and monitoring of the virus in humans, animals, and the environment are critically important for protecting public health from the disease.
In collaboration with Dr. Wu in the EECS department, Eda’s laboratory has been working on the development of rapid on-site pathogen detection methods, which resulted in nearly 20 publications, major federal funding (NSF, DHS), two patents, several license/research agreements with multiple private companies in the last 10 years.
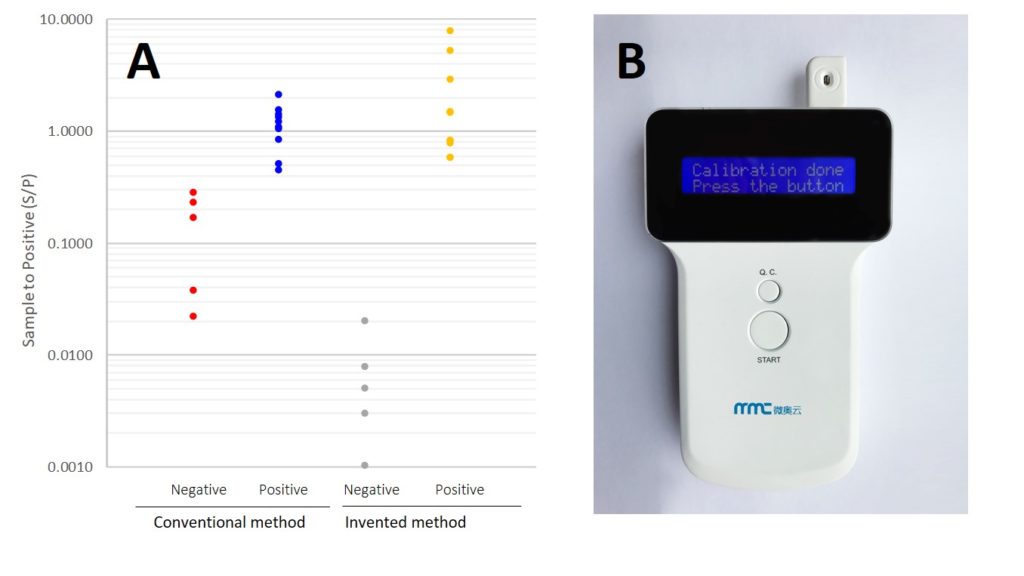
Researcher: Chunlei Su, Department of Microbiology
Most emerging infectious diseases in humans are zoonoses. Among zoonotic pathogens, the protozoan parasite Toxoplasma gondii is one of the most wide spread microorganisms. It infects a broad range of hosts including mammals and birds. Toxoplasma gondii has been recognized as a major food- and water-borne pathogen in humans due to frequent outbreaks of infections. One-third of the human population worldwide is chronically infected. Infections in healthy pregnant women may cause blindness, intellectual disabilities, or even death of the fetuses. Reactivation of latent infection in immunocompromised patients, such as individuals with AIDS, can cause life-threatening encephalitis. Infection of T. gondii in sensitive animals such as sheep cab cause abortion, and high mortality in marsupials and New World monkeys. Genetic studies from global samples elucidated high diversity in T. gondii parasites and the isolates vary markedly in virulence. Severe, acute, disseminated toxoplasmosis occurs in immunocompetent human patients when infected with unique isolates derived from wildlife sources.
To better understand major reservoirs and transmission patterns of this parasite, my laboratory focuses on large-scale molecular epidemiology and population genetics of T. gondii. We developed a multiplex nested PCR-RFLP method to identify T. gondii collected from animals and humans worldwide, revealed subpopulation structures in different continents, as well as worldwide distribution of a few clonal lineages of T. gondii. Genetic diversity from our studies elucidated how this population is structured and how human activities have shaped the transmission and evolution of this parasite. To prevent diseases caused by this parasite, we are investigating cell-culture based system to study life cycle of T. gondii and the long term goal is to develop vaccine to block its transmission.
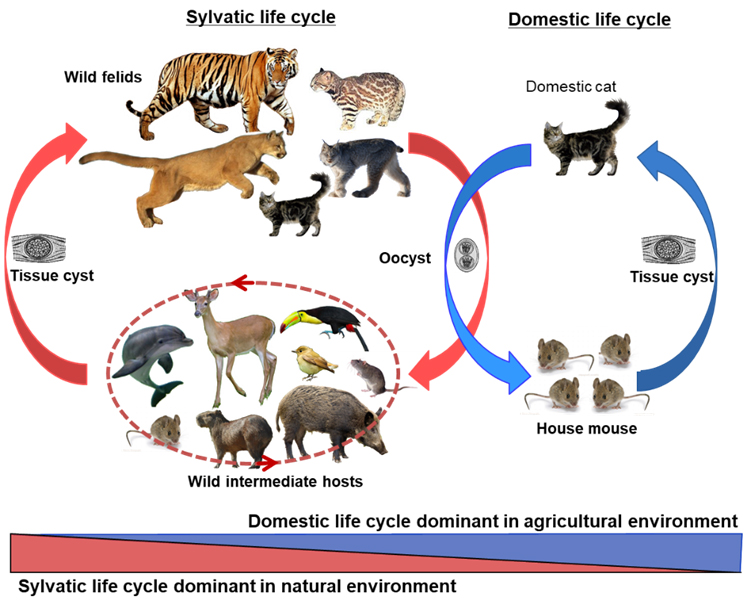
Animal Health
Researchers: Emily Martin, Department of Medicine
Dan Grove, Department of Forestry, Wildlife & Fisheries
Shigetoshi Eda, Department of Forestry, Wildlife & Fisheries
Jayne Wu, Department of Electric Engineering and Computer Science
Jennifer DeBruyn, Department of Biosystems Engineering and Soil Science
Chronic Wasting Disease (CWD) is a progressive, fatal disease that affects the brain, spinal cord, and many other tissues of farmed and free-ranging deer, elk, and moose (cervids). Currently the known CWD endemic area in Tennessee is spread out over roughly 4,500 square miles, which equates to approximately 2.9 million acres. The majority of the land is privately owned and managed property; therefore, the crux of successful CWD management depends on engaging concerned stakeholders such as landowners and hunters. Wildlife managers can work with landowners to identify both natural areas where deer have congregated and artificial congregation areas and combine that data with GPS locations from collared deer.
The primary goal of this collaborative effort is to develop a testing platform and a sample treatment protocol specifically for detecting CWD prions in soil samples. We will focus testing on these areas to identify infectious hot spots. Once hot spots are identified using the environmental testing, landowners will be given a suite of land management techniques to begin to mitigate these locations as secondary disease transmission sites. Follow-up testing can then be used to determine if the applied management was effective or if additional methods are needed.
Researcher: Charles Sims, Howard H Baker Jr Center for Public Policy
Wildlife diseases pose a substantial threat to the provisioning of ecosystem services. We use a novel modeling approach to study the potential loss of these services through the imminent introduction of chronic wasting disease (CWD) to elk populations in the Greater Yellowstone Ecosystem (GYE). A specific concern is that concentrating elk at feedgrounds may exacerbate the spread of CWD, whereas eliminating feedgrounds may increase the number of elk on private ranchlands and the transmission of a second disease, brucellosis, from elk to cattle. To evaluate the consequences of management strategies given the threat of two concurrent wildlife diseases, we develop a spatiotemporal bioeconomic model. GPS data from elk and landscape attributes are used to predict migratory behavior and population densities with and without supplementary feeding. We use a 4,800 km2 area around Pinedale, Wyoming containing four existing feedgrounds as a case study. For this area, we simulate welfare estimates under a variety of management strategies.
Our results indicate that continuing to feed elk could result in substantial welfare losses for the case‐study region. Therefore, to maximize the present value of economic net benefits generated by the local elk population upon CWD’s arrival in the region, wildlife managers may wish to consider discontinuing elk feedgrounds while simultaneously developing new methods to mitigate the financial impact to ranchers of possible brucellosis transmission to livestock. More generally, our methods can be used to weigh the costs and benefits of human‐wildlife interactions in the presence of multiple disease risks.
Researcher: Matt Gray and Deb Miller, Department of Forestry, Wildlife and Fisheries
Batrachochytrium salamandrivorans (Bsal) is a fungal pathogen that likely originated in Asia, where local species harbor infections without signs of clinical disease. Bsal has recently been documented in Europe, where it is causing mass die-offs of some salamander species. It is hypothesized to have been introduced through unregulated international trade. To date, Bsal has not been detected in amphibian populations in North America. However, North America is a global hotspot for salamander biodiversity, accounting for 50 percent of species worldwide, so there is substantial concern about the possibility of Bsal’s introduction to the continent.
Get more information at the Bsal Project website.
Ticks and their pathogens are expanding their ranges in North America which includes Tennessee and the southeastern United States. This is a significant concern because ticks transmit an array of pathogens and cause toxic conditions, affecting a variety of host species. Contact between companion animals, livestock, and wildlife is a frequent occurrence and provides opportunities for spillover effects to humans and other animals. We investigate the life histories of ticks and their pathogens to develop prevention, detection, and response strategies for infected-ticks capable of transmitting pathogens or whose blood-feeding behaviors cause expensive economic effects. This is documented with the blacklegged tick (Ixodes scapularis), whose immature life stages feed on hosts such as white-footed mouse or lizards and adult stage feed on white-tailed deer and other larger mammals. Understanding these relationships help to explain why managing mice will curb Lyme disease cases (natural reservoir for Borrelia burgdorferi)and managing deer will curb tick populations.
Recently, the Asian longhorned tick (Haemaphysalis longicornis) was discovered in East Tennessee. This is important because this tick species is an economic threat to agricultural industries (e.g., blood-feeding populations can cause anemia and individuals can transmit a variety of pathogens). Since many tick species are expanding into the region, we are developing a comprehensive tick surveillance program, to detect ticks as soon as possible and use those collections to develop educational material for stakeholders and researchers. In addition, this research aims to identify predictors for detection and assess management options. Understanding the relationships between host life histories, particularly those transfigured due to human behavior, are important considerations for health and the transmission of tick-borne pathogens.


Researcher: Yang Zhao, Department of Animal Science
Global farm animal production soared in the past century to meet the increasing demands on protein resources and is projected to keep growing in decades to come.
Zhao and his team have been working on precision livestock farming (PLF) which addresses the environmental and animal health challenges that result from the expansion of animal production by using sensors, computer vision, artificial intelligence, and robots.
Zhao also spearheads studies regarding airborne transmission of animal-related pathogens and the mitigation solutions. His ongoing projects include automatic monitoring poultry walking ability using computer vision and machine learning; evaluating welfare and behavior of broilers (meat-type poultry) at different growth rate; improving laying behavior of hens using robot in cage-free housing system; and determining concentration and prevalence of airborne E. coli in poultry houses.

Detection of broilers using computer vision and machine learning. 
Training the laying behavior of hens using a robot. 
Sampling of airborne avian influenza virus during 2015 outbreak 
Feather coverage evaluation using thermography. 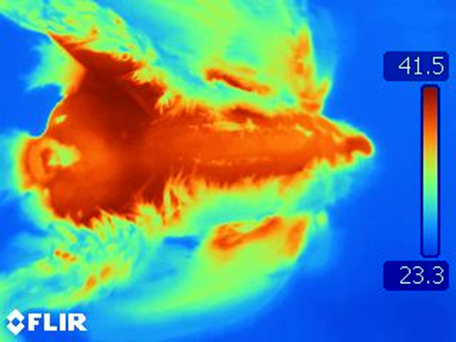
Feather coverage evaluation using thermography. 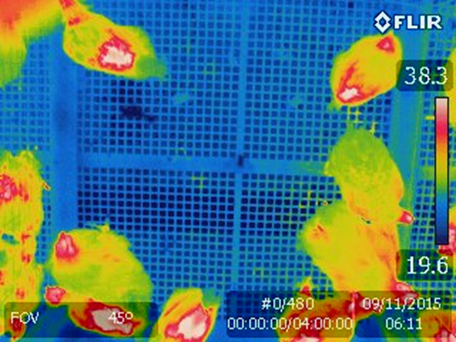
Feather coverage evaluation using thermography.
Plant Health
Researcher: Denita Hadziabdic Guerry, Department of Entomology & Plant Pathology
Thousand Cankers Disease (TCD) complex, Geosmithia morbida, vectored by the walnut twig beetle, Pityophthorus juglandis, has been associated with disease outbreaks in walnut trees, Juglans spp. The unique relationship of the host-pathogen-vector complex can result in significant evolutionary pressure for the pathogen, thus shaping the genome of G. morbida in unique ways that have not yet been documented in fungi. We are investigating a genome-enabled approach focused on G. morbida that will provide a better understanding of the biology of the pathogen involved in TCD and identify candidate genes and functions required for pathogenesis. Most evolutionary studies have focused on pathogens of crops that are not insect vectored, making this research a unique contribution in determining co-evolutionary history, and shedding light on emergence of new pathogenic fungi that continue to be a threat to fruit, nut, and forest trees worldwide.
Researcher: Denita Hadziabdic Guerry, Department of Entomology & Plant Pathology
An additional component of Dr. Guerry’s research program focuses on conservation efforts of native trees, preservation of biodiversity in North American forests, and overall forest and tree health and protection on a global scale. They intend to devote research and teaching efforts to limit the impact of forest diseases and provide an understanding of the evolutionary dynamics, diversity and disease ecology of environmentally, socially and economically, significant trees. Currently, her lab is involved in a number of conservation genetics projects focused on endangered and endemic plants in the US and Ghana, Africa, including food security crops. In that capacity, the lab is involved with conservation efforts of Stewartia ovata ( mountain Stewartia) an endemic tree native to the Appalachian Mountains; Helianthus verticillatus (whorled sunflower), federally endangered plants species in Tennessee, Alabama, and Georgia; and the biodiversity and preservation of an antimalarial plant endemic to Ghana, Cryptolepis sanguinolenta.
Environmental Health
Researcher: Laura Russo, Department of Entomology & Plant Pathology
The human gut microbiome plays a role in health and may represent a key nutritional symbiosis, but it is difficult to study microbial symbionts in the human gut because humans are omnivores with changeable diets, leading to complex nutritional inputs and needs. On the other hand, insects with specialized diets provide a useful study system for questions addressing the role of nutritional symbionts: their diet is comparatively simple and can be manipulated experimentally. Bees, a monophyletic clade of insects specialised on plant pollens, can be used as a study system to explore the role of symbionts in nutrition.




Researcher: Laura Russo, Department of Entomology & Plant Pathology
It has been clearly established that dietary nutrition plays a key role in human health, relating to decreased risk for a variety of diseases (WHO 2003), increased resistance to disease (Lock et al 2005), and also to the health and diversity of our gut flora (Wu et al 2011). On the other hand, we are only just beginning to understand the role of nutrition in bee health. It is clear that bees require a diverse and nutritious diet (e.g. Di Pasquale et al 2013), just as we do, but what elements of nutrition are required to promote healthy pollinators? This project aims to evaluate the nutritional properties of pollinator-attractive native plant species in an attempt to provide evidence-based recommendations on planting pollinator gardens that best fill the nutritional requirements of wild and managed pollinators.




Researcher: Laura Russo, Department of Entomology & Plant Pathology
Pollinators contribute important services in any ecosystem, including wetlands. At the same time, habitats such as wetlands provide a bounty of flowering plant species that promote pollinator populations. For some pollinating insects, such as flower flies, wetlands may be an essential part of their life cycle. To better understand the mutually beneficial relationship between wetland habitats and pollinating insects, we will survey interactions between the flowering plant community and pollinator community in wetlands across a gradient from less disturbed (remnant) to more disturbed (restored). We will also measure wetland quality through a variety of methods, including light exposure, water quality, soil pH, and land-use surrounding the wetlands. We expect to find greater plant and pollinator species richness in wetlands that are less disturbed and have a higher water quality. We also expect to find that the pollinators which react most strongly to wetland quality will be species of flower flies that rely on wetlands for the larval stage of their life cycle.

